Hydrogen can be produced from biogas through various reforming processes, including steam reforming, dry reforming, and autothermal reforming. These methods involve reacting biogas, which is primarily methane and carbon dioxide, with steam or other reactants at high temperatures to produce a mixture of hydrogen and carbon monoxide known as syngas.
The carbon monoxide can then be converted to additional hydrogen via the water-gas shift reaction.
Key Takeaways for Hydrogen Production from Biogas
- Biogas reforming is a promising process for producing green hydrogen that reduces dependence on natural gas while cutting greenhouse gas emissions.
- The composition of biogas (typically 40-65% methane and 35-55% carbon dioxide) significantly impacts the efficiency and yield of hydrogen production.
- Four major reforming technologies—steam reforming, dry reforming, autothermal reforming, and partial oxidation—each offer distinct advantages for converting biogas to hydrogen.
- Catalyst selection is crucial for efficient hydrogen production, with nickel-based catalysts being cost-effective while noble metal catalysts provide superior performance.
- Advanced purification methods, including membrane separation and pressure swing adsorption, are essential for producing high-purity hydrogen suitable for fuel cells and industrial applications.
Converting biogas to hydrogen represents one of the most promising pathways for sustainable energy production. This renewable approach not only produces clean hydrogen but also gives purpose to biogas that might otherwise contribute to greenhouse gas emissions.

Several companies have been pioneering innovative methods to maximise hydrogen yield from biogas, helping communities transition to cleaner energy alternatives while reducing carbon footprints.
There is big money at stake for any business that gains the lead in this technology. Just consider the fact that Sustainable Aviation Fuel (SAF), although emerging as the transition fuel for aviation, can only be considered a transitional fuel.
SAF cannot eliminate the non-carbon dioxide greenhouse gas effects caused by hydrocarbon combustion, such as low levels of:
- soot
- water vapour
- sulphur aerosols and
- contrails
This amounts to a net aviation anthropological warming contribution of 4% (CircularOnline Spring 2025), and therefore, the airlines will be forced to accelerate the move to hydrogen fuel for new aircraft. This, even if the move to SAF production and use without blending with traditional fuel proves successful.
The growing interest in hydrogen as an energy carrier stems from its versatility and zero-emission combustion properties.
When produced from biogas—a renewable resource generated from organic waste decomposition—hydrogen becomes part of a circular economy that transforms waste into valuable energy. This process offers dual environmental benefits: waste management and clean fuel production.
Biogas to Hydrogen: A Renewable Energy Breakthrough
Biogas reforming represents a significant breakthrough in renewable hydrogen production. Unlike hydrogen derived from fossil fuels, biogas-sourced hydrogen qualifies as green hydrogen when the process is powered by renewable energy. This pathway provides a sustainable alternative to conventional hydrogen production methods that rely heavily on natural gas and coal, which together account for nearly 95% of current hydrogen production worldwide.
The technology bridges two critical aspects of the energy transition: waste management and clean fuel production. Agricultural waste, food scraps, sewage, and landfill gas—all sources of methane emissions if left untreated—become valuable feedstock for hydrogen production. This transformation converts potential environmental pollutants into a zero-emission fuel that can power everything from vehicles to industrial processes.
What makes this approach particularly appealing is its scalability. Systems can be designed to serve small rural communities utilising agricultural waste or scaled up to process municipal solid waste from large urban centres. The flexibility allows for distributed hydrogen production networks that reduce transportation costs and increase energy security.
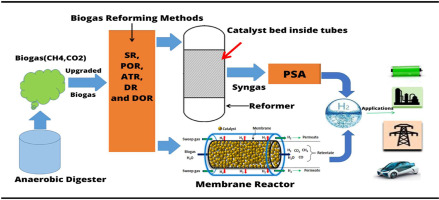
“biogas reforming …” from www.sciencedirect.com and used with no modifications.
The Science Behind Biogas Composition
Understanding biogas composition is fundamental to optimising hydrogen production processes. The exact makeup varies based on the organic source material, digestion conditions, and collection methods, creating unique challenges for reforming technologies.
What Makes Up Biogas and Why It Matters
Biogas primarily consists of methane (CH₄) and carbon dioxide (CO₂), with smaller amounts of nitrogen, oxygen, hydrogen sulfide, ammonia, and siloxanes. The methane content typically ranges from 40-65%, while carbon dioxide makes up about 35-55%. This composition significantly influences reforming efficiency, as methane serves as the primary hydrogen source while carbon dioxide can either participate in the reforming process or act as a diluent. The presence of trace compounds, particularly sulfur-containing gases, can profoundly impact catalyst performance and system longevity—even at concentrations of just a few parts per million.
Methane Content and Energy Potential
The methane percentage in biogas directly correlates with its hydrogen production potential. Higher methane content translates to greater hydrogen yield since each methane molecule can theoretically produce up to four hydrogen atoms through steam reforming. Biogas from landfills typically contains 45-55% methane, while agricultural digesters can produce biogas with 55-65% methane content.
Feedstock selection significantly influences this crucial metric. For instance, food waste typically generates biogas with higher methane content than wastewater sludge. Some advanced digestion systems even implement two-stage processes to optimise methane production, thereby enhancing subsequent hydrogen yields during reforming.
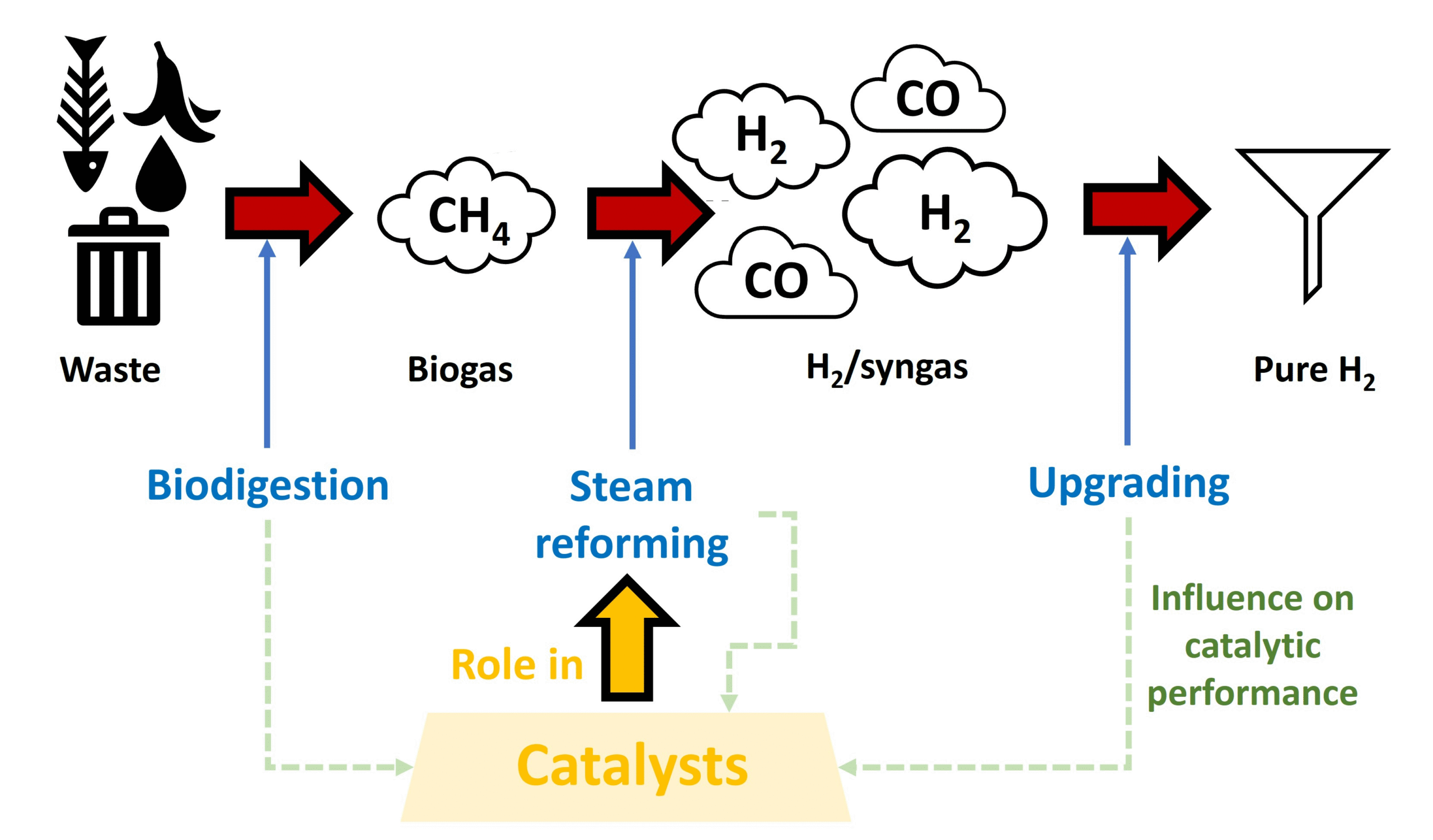
“Catalysis for Biogas Steam Reforming” from www.mdpi.com and used with no modifications.
Common Contaminants That Affect Hydrogen Production
Contaminants in biogas present significant challenges to hydrogen production efficiency. Hydrogen sulfide (H₂S), even at concentrations of 100-10,000 ppm, causes rapid poisoning of reforming catalysts by forming metal sulfides that block active sites. This sulfur poisoning can reduce catalyst activity by up to 60% within hours of operation if left untreated.
Siloxanes, commonly found in landfill gas and wastewater biogas, deposit as silica during combustion or reforming, coating catalysts and heat exchangers with an abrasive layer that reduces efficiency and increases maintenance requirements. Ammonia, while less problematic during reforming, can cause issues in downstream purification systems by competing for adsorption sites in pressure swing adsorption units.
Biogas Composition Comparison by Source
Landfill Gas: 45-55% CH₄, 30-40% CO₂, high siloxanes, variable H₂S
Agricultural Waste: 55-65% CH₄, 35-45% CO₂, moderate H₂S, low siloxanes
Wastewater Treatment: 60-70% CH₄, 30-40% CO₂, high H₂S, moderate siloxanes
Food Waste: 65-75% CH₄, 25-35% CO₂, variable H₂S, low siloxanes
4 Major Reforming Technologies That Convert Biogas to Hydrogen
The transformation of biogas into hydrogen relies on various reforming technologies, each with distinct operational principles, efficiency profiles, and suitability for different scales of production. The selection of the appropriate reforming method depends on factors including biogas composition, desired hydrogen purity, energy integration possibilities, and economic considerations.
1. Steam Reforming: The Industry Workhorse
Steam reforming stands as the most widely employed method for hydrogen production from biogas. This endothermic process combines methane with steam at high temperatures (700-900°C) over a catalyst to produce hydrogen and carbon monoxide. The primary reaction (CH₄ + H₂O → CO + 3H₂) is typically followed by the water-gas shift reaction (CO + H₂O → CO₂ + H₂), which further increases hydrogen yield by converting carbon monoxide with additional steam.
The main advantage of steam reforming lies in its high hydrogen yield—up to 4 moles of hydrogen per mole of methane is theoretically possible. Commercial systems typically achieve 70-80% of this theoretical maximum, making it the most efficient reforming technique for hydrogen production. The process benefits from decades of industrial optimisation, with well-established catalysts and reactor designs that deliver reliable performance at scale.
However, steam reforming faces challenges when applied to biogas rather than natural gas. The high CO₂ content in biogas dilutes the methane, reducing per-volume hydrogen yield and requiring larger reactors. Additionally, the substantial energy input needed to maintain reaction temperatures means efficient heat integration is crucial for economic viability, especially at smaller scales.
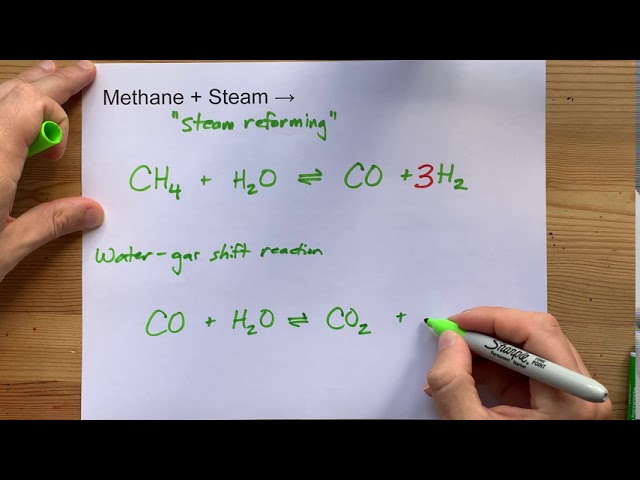
“Methane + Steam – Steam Reforming” from www.youtube.com and used with no modifications.
2. Dry Reforming: Utilising CO2 in Biogas
Dry reforming presents a unique approach specifically suited to biogas by leveraging its high CO₂ content as a reactant rather than viewing it as a contaminant. The process reacts methane with carbon dioxide (CH₄ + CO₂ → 2CO + 2H₂) at temperatures between 650-1000°C to produce syngas with a lower H₂:CO ratio than steam reforming. This method effectively utilises both primary biogas components, eliminating the need for CO₂ separation in many applications.
The process offers compelling environmental benefits by consuming two greenhouse gases simultaneously. For biogas producers, dry reforming eliminates costly CO₂ removal steps that would otherwise be necessary for biomethane production. The lower hydrogen yield (2 moles H₂ per mole CH₄ compared to 4 in steam reforming) is offset by the simplified pretreatment and the ability to operate with raw biogas streams.
The main technical challenge facing dry reforming is carbon deposition (coking) on catalysts, which occurs more readily than in steam reforming due to the carbon-rich environment. Advanced catalyst formulations using promoters like cerium, lanthanum, and potassium have shown promise in mitigating this issue by enhancing carbon gasification reactions.
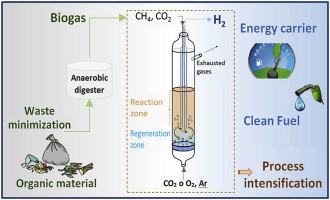
“Dry reforming of biogas in a fluidised bed reactor…” from www.sciencedirect.com and used with no modifications.
3. Autothermal Reforming: Balancing Heat Requirements
Autothermal reforming (ATR) combines elements of both steam reforming and partial oxidation to create a thermally self-sustaining process. By introducing controlled amounts of oxygen alongside steam and methane, the exothermic partial oxidation reaction (CH₄ + ½O₂ → CO + 2H₂) provides the heat required for the endothermic steam reforming reaction. This balanced approach eliminates the need for external heating, simplifying reactor design and improving energy efficiency.
For biogas applications, ATR offers particular advantages in modular and distributed systems where heat management presents challenges. The process typically operates at temperatures between 800-1100°C with shorter residence times than conventional steam reforming, allowing for more compact reactor designs. The resulting syngas has an H₂:CO ratio between 1.5 and 2.5, making it suitable for both hydrogen production and synthesis of liquid fuels.
The primary drawback is the requirement for pure oxygen or air, which introduces either additional costs for oxygen separation or nitrogen dilution when using air. Nevertheless, ATR's thermal efficiency and operational flexibility make it increasingly attractive for medium-scale biogas conversion, particularly when coupled with effective downstream purification systems.
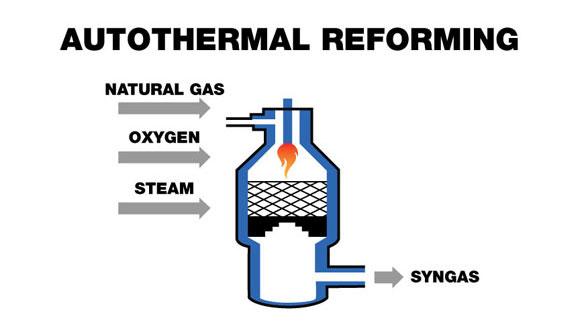
“Auto-Thermal Reforming – Global Syngas …” from globalsyngas.org and used with no modifications.
4. Partial Oxidation: Quick Conversion with Lower Hydrogen Yield
Partial oxidation (POX) offers the simplest approach to hydrogen production from biogas, directly reacting methane with limited oxygen to produce hydrogen and carbon monoxide (CH₄ + ½O₂ → CO + 2H₂). Unlike other reforming methods, POX can operate without catalysts at temperatures exceeding 1200°C, though catalytic versions running at 800-900°C are more common in biogas applications.
The exothermic nature of partial oxidation eliminates external heating requirements, enabling rapid startup and flexible operation—qualities particularly valuable for intermittent biogas sources. The process produces a hydrogen-rich syngas with minimal steam requirements, reducing water consumption and simplifying system design. These characteristics make POX particularly suitable for remote installations or facilities with limited infrastructure.
The hydrogen yield in POX (2 moles H₂ per mole CH₄) is half that of steam reforming, representing its main disadvantage. Additionally, the high temperatures in non-catalytic versions lead to significant thermal efficiency losses, and the process produces syngas with higher levels of carbon monoxide, requiring more intensive downstream purification. Despite these limitations, POX remains valuable in niche applications where simplicity and robustness outweigh maximum hydrogen yield.
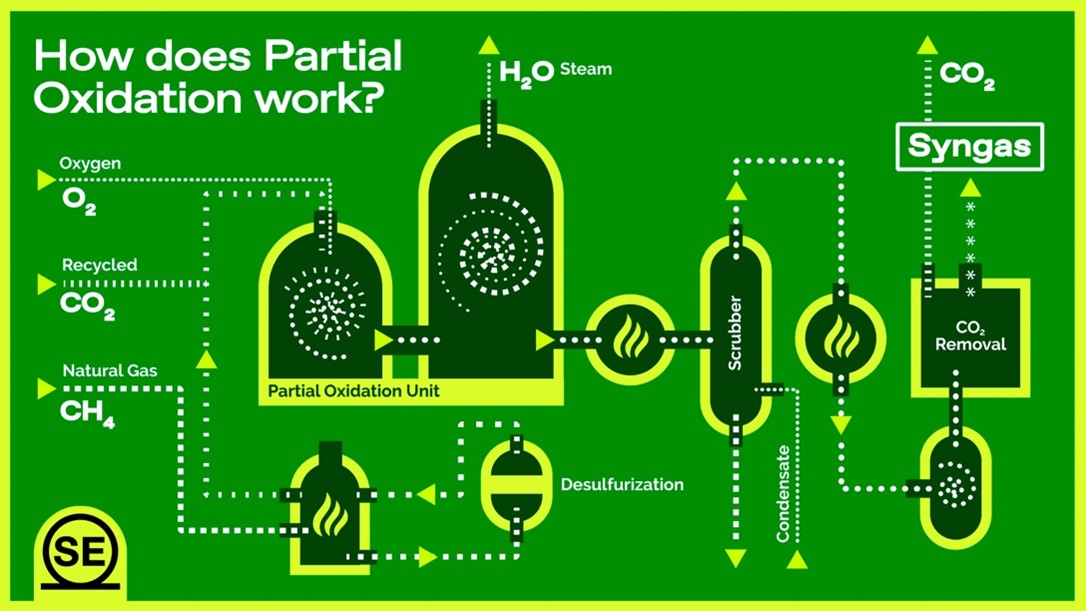
“Partial Oxidation Syngas Production Schematic diagram – Multi-Discipline …” from www.samuelengineering.com and used with no modifications.
Catalyst Science: The Heart of Efficient Reforming
Catalysts fundamentally determine the economic viability of biogas-to-hydrogen processes by influencing reaction temperatures, conversion rates, and operational longevity. The right catalyst formulation can reduce energy requirements by over 30% while significantly extending maintenance intervals, directly impacting production costs and system reliability.
Nickel-Based Catalysts and Their Performance
Nickel-based catalysts dominate commercial biogas reforming due to their exceptional balance of cost and performance. Typically consisting of 5-25% nickel dispersed on support materials like alumina, silica, or zirconia, these catalysts facilitate methane activation at temperatures as low as 650°C. Their widespread industrial adoption has been driven by nickel's ability to effectively break C-H bonds while maintaining sufficient activity for thousands of operational hours.
Support materials play a crucial role in catalyst performance, with recent formulations incorporating mixed oxides like MgAl₂O₄ or CeZrO₂ to enhance thermal stability and resistance to carbon formation. Promoters such as potassium, lanthanum, and cerium are often added in small quantities (0.5-3%) to improve nickel dispersion and facilitate carbon gasification reactions. The industrial standard Ni/Al₂O₃ catalyst achieves methane conversion rates of 85-95% under optimal conditions, though performance degrades more rapidly when processing biogas compared to natural gas due to contaminant exposure.
Noble Metal Catalysts: When Higher Costs Meet Better Results
Noble metal catalysts based on platinum, rhodium, and ruthenium represent the premium tier of reforming catalysts, offering exceptional performance under challenging conditions. These metals demonstrate superior resistance to sulfur poisoning, with platinum catalysts maintaining activity at H₂S concentrations up to 5 ppm—approximately ten times higher than conventional nickel systems. The enhanced carbon resistance of rhodium catalysts makes them particularly valuable for dry reforming of biogas, where carbon deposition presents a major challenge.
Despite comprising only 0.1-2% of the catalyst by weight, these precious metals deliver significantly higher per-site activity than nickel alternatives. Ruthenium catalysts, for instance, can achieve complete methane conversion at temperatures 50-100°C lower than nickel catalysts with similar metal loading. This temperature reduction translates to substantial energy savings in continuous operations, partially offsetting the higher initial catalyst cost.
The economic equation typically limits noble metal catalysts to specialised applications where their unique properties justify the 5-10x cost premium over nickel-based alternatives. These include small-scale distributed systems where reliability outweighs material costs, applications processing biogas with fluctuating contaminant levels, and high-pressure reforming where catalyst volume significantly impacts system design and capital expenditure.
Catalyst Deactivation Problems and Solutions
Catalyst deactivation represents the primary operational challenge in biogas reforming, with three main mechanisms shortening catalyst lifespan: coking (carbon deposition), sintering (thermal degradation), and poisoning (chemical contamination). Carbon formation occurs when methane or carbon oxides decompose on the catalyst surface, blocking active sites and potentially damaging the catalyst structure. This process accelerates in dry reforming environments and at lower steam-to-carbon ratios.
Sintering becomes significant at temperatures above 700°C, causing metal crystallites to migrate and coalesce, reducing available surface area by up to 50% after 1000 hours of operation. Biogas contaminants represent the most insidious deactivation mechanism—sulfur compounds bind strongly to active sites, while siloxanes deposit silica layers that permanently encapsulate catalyst particles.
Modern catalyst formulations address these challenges through structural and compositional innovations. Bimetallic formulations like Ni-Co and Ni-Fe demonstrate enhanced coke resistance by modifying carbon binding energies. Core-shell structures with protective oxide layers mitigate sintering by constraining metal particle mobility. For contaminant resistance, specialised guard materials upstream of the main catalyst absorb sulphur and silicon compounds, extending catalyst lifetime from months to years in properly designed systems.
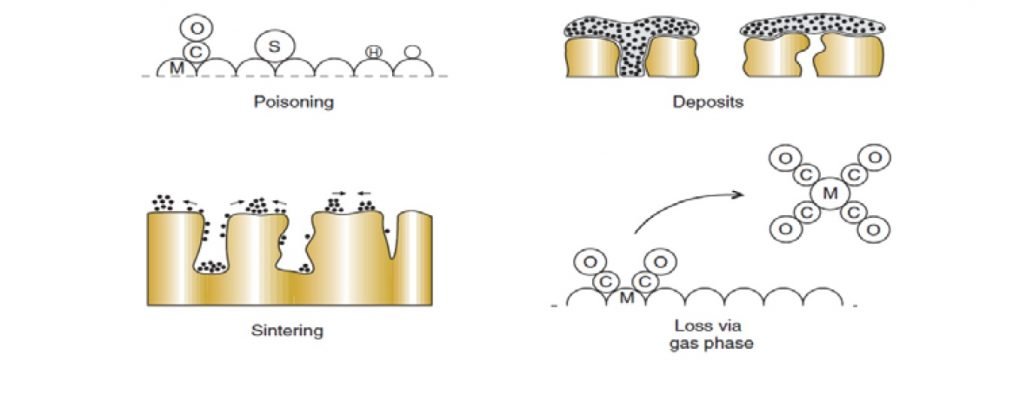
“Catalyst deactivation Common causes …” from ammoniaknowhow.com and used with no modifications.
Purification Methods That Deliver High-Quality Hydrogen
The reforming process generates hydrogen mixed with carbon dioxide, carbon monoxide, unreacted methane, and trace contaminants. Purification is essential to meet application requirements, with fuel cells typically demanding 99.99% hydrogen purity. The choice of purification technology significantly impacts both capital costs and operational efficiency of biogas-to-hydrogen systems.
Membrane Separation Technology
Hydrogen-selective membranes offer an elegant approach to purification, allowing hydrogen molecules to permeate while blocking larger gas species. Palladium and palladium-alloy membranes achieve the highest selectivity, operating on a solution-diffusion mechanism where only hydrogen dissolves into and passes through the metallic structure. These membranes can deliver ultra-pure hydrogen (99.9999%) in a single step, though their high cost (typically $3,000-10,000 per square meter) limits application to smaller systems or high-value applications.
Polymeric membranes present a more economical alternative, utilising materials like polyimide, polysulfone, and perfluoropolymers to achieve separation through size-selective diffusion. While less selective than metallic alternatives, modern polymer membranes achieve hydrogen purities of 95-98% with significantly lower investment costs. Ceramic and carbon molecular sieve membranes occupy a middle ground, offering improved temperature stability (up to 600°C) that allows for potential integration directly into the reforming process as membrane reactors, simultaneously enhancing conversion and performing separation.
The most significant recent advancement in membrane technology has been the development of thin-film composite membranes that combine a thin selective layer (0.1-1 μm) with a porous, mechanically robust support. This approach reduces precious metal requirements by over 90% while maintaining separation performance, dramatically improving economic viability for medium-scale applications processing 50-500 kg of hydrogen daily.
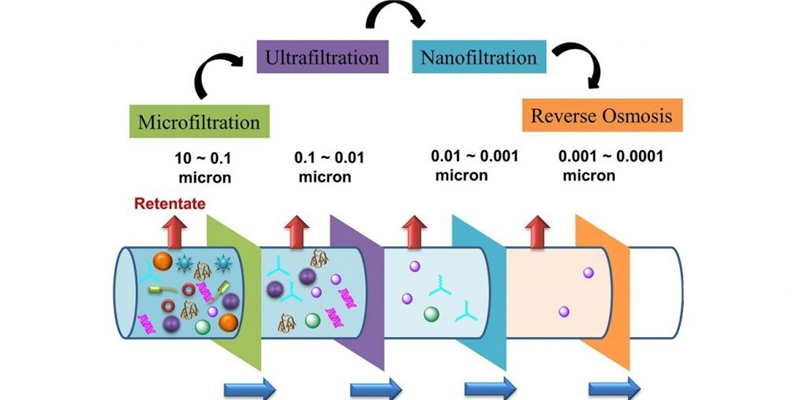
“Membrane Separation Processes …” from www.hongtekfiltration.com and used with no modifications.
Pressure Swing Adsorption (PSA) Systems
Pressure Swing Adsorption represents the industry standard for hydrogen purification at a commercial scale, accounting for over 85% of hydrogen purification capacity worldwide. PSA systems utilise materials like activated carbon, zeolites, and carbon molecular sieves that preferentially adsorb impurities under pressure while allowing hydrogen to pass through. When the adsorbent approaches saturation, pressure is reduced to release captured impurities in a regeneration cycle.
Modern PSA systems typically employ 4-16 adsorption vessels operating in staggered cycles to provide continuous hydrogen output. This configuration achieves hydrogen recoveries of 70-90% at purities exceeding 99.999%, suitable for even the most demanding applications, including PEM fuel cells. The technology scales efficiently from 100 kg/day to over 100,000 kg/day, with larger systems benefiting from improved energy efficiency and reduced capital costs per unit of capacity.
For biogas-derived syngas, PSA systems require careful design considerations to handle the high CO₂ content. Multi-layer adsorbent beds typically incorporate both activated carbon (for CO₂ removal) and zeolites (for CO and N₂ removal) to optimise performance. Advanced control systems that adjust cycle times and pressure levels based on feed composition have improved hydrogen recovery from biogas-derived syngas by 5-15% compared to fixed-parameter operation.
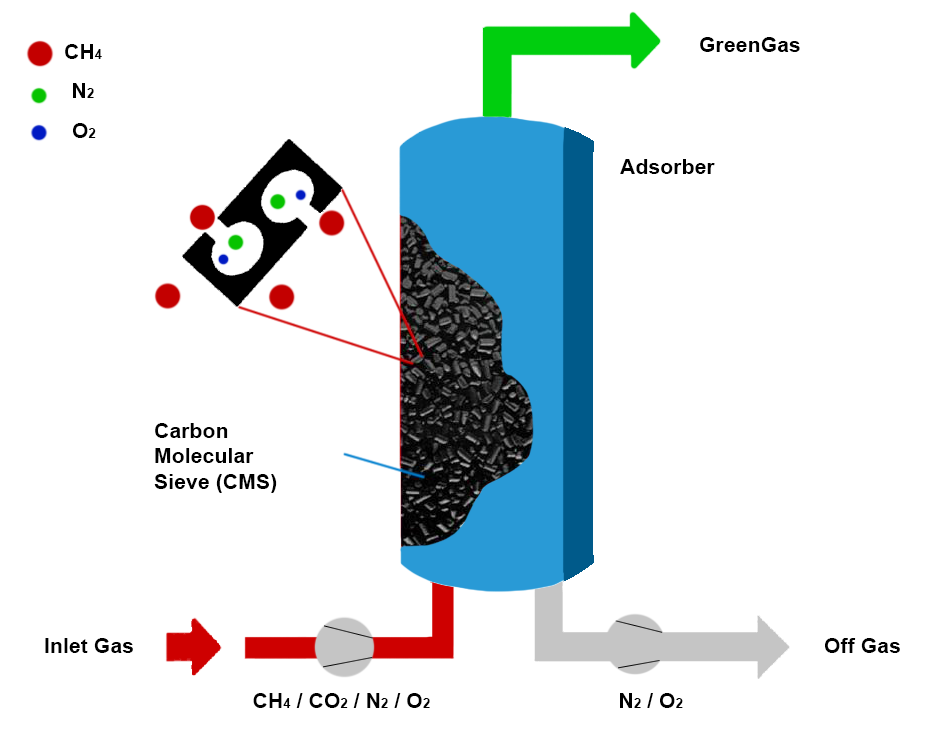
“Pressure Swing Adsorption (PSA) Schematic …” from www.apexgasgenerators.com and used with no modifications.
Cryogenic Distillation for Ultra-Pure Hydrogen
Cryogenic distillation leverages the different boiling points of gases to achieve separation, cooling the gas mixture to temperatures where components condense at different stages. While rarely used as the primary purification method for hydrogen due to its high energy consumption, cryogenic techniques excel at recovering valuable byproducts like carbon monoxide and liquid carbon dioxide from the purification process.
The process operates by cooling syngas to temperatures below -150°C, where most impurities condense while hydrogen remains gaseous. This approach achieves exceptional purity levels (99.9999+%) and can simultaneously produce instrument-grade carbon monoxide and food-grade liquid CO₂, potentially generating additional revenue streams. The primary limitation is scale—cryogenic systems typically become economical only at processing capacities above 5,000 kg H₂/day.
When processing biogas-derived syngas with high CO₂ content, cryogenic systems offer unique advantages by efficiently separating and liquefying this major component. Some integrated facilities combine PSA for bulk hydrogen purification with selective cryogenic processing of PSA tail gas to maximise resource recovery and improve overall system economics.
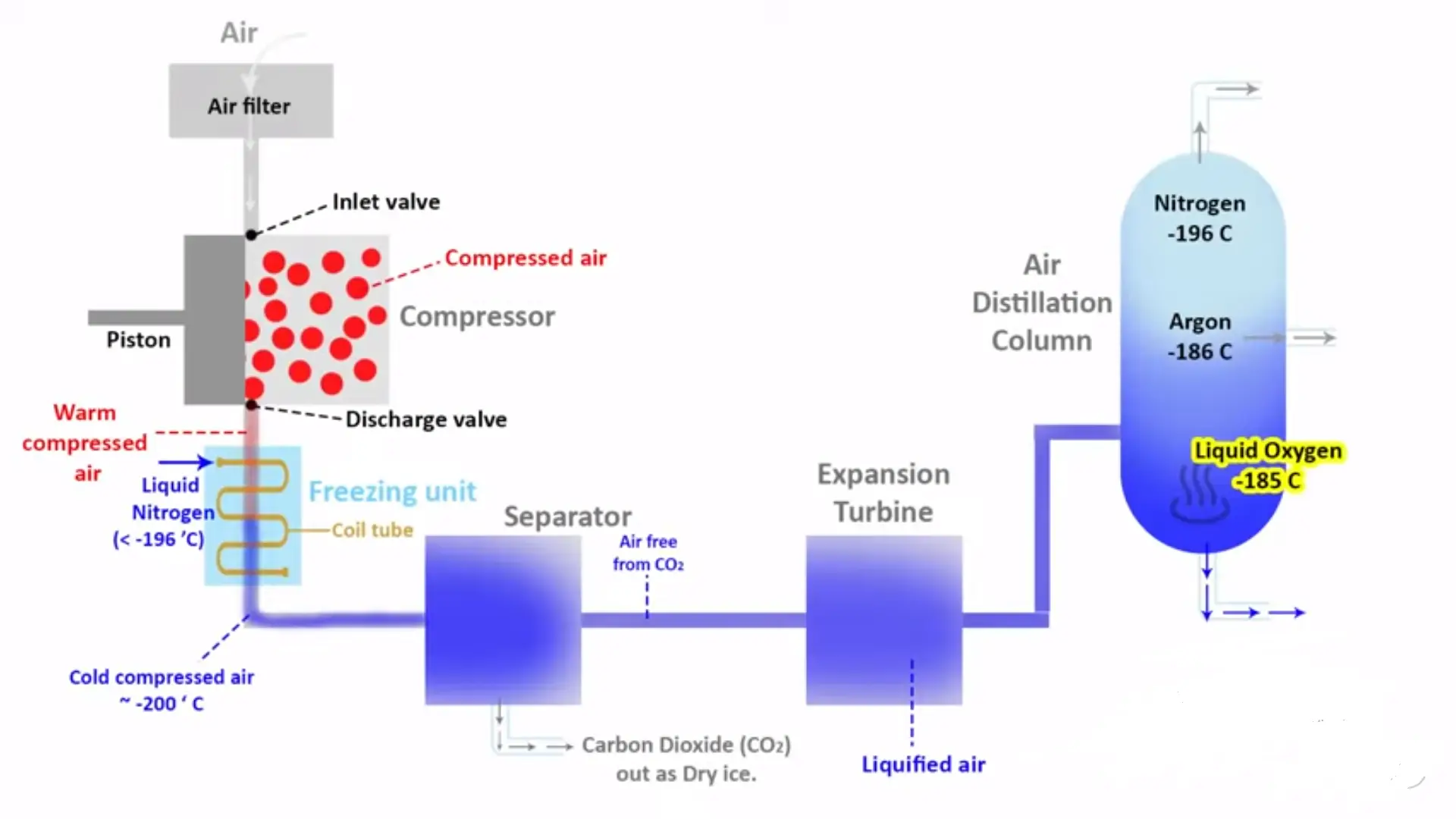
“Cryogenic Distillation Process: A Guide …” from www.jalonzeolite.com and used with no modifications.
Removing Sulfur and Other Contaminants
Contaminant removal represents a critical pretreatment step that protects both reforming catalysts and downstream purification systems. Sulfur compounds, primarily hydrogen sulfide (H₂S) and organic sulfides, are typically removed using zinc oxide beds that convert sulfur compounds to solid zinc sulfide through chemisorption. This approach effectively reduces sulfur concentrations from 1000+ ppm to below 50 ppb, necessary for preventing catalyst poisoning.
Siloxanes, particularly problematic in landfill and wastewater biogas, require specialised removal techniques. Activated carbon impregnated with metal ions demonstrates high capacity for siloxane adsorption, while newer polymeric adsorbents offer improved regeneration characteristics. For comprehensive contaminant management, multi-stage cleaning systems may incorporate refrigeration steps to condense volatile compounds, followed by targeted adsorbent beds for specific contaminant classes.
Emerging technologies for contaminant removal include regenerable metal-organic frameworks (MOFs) that offer 3-5 times higher sulfur capacity than conventional adsorbents, and plasma-assisted catalytic systems that decompose complex contaminants into more easily managed compounds. These advanced approaches are particularly valuable for processing variable-quality biogas, where contaminant levels may fluctuate significantly based on feedstock and digestion conditions.
Energy Integration Strategies for Maximum Efficiency
Energy integration fundamentally determines the economic viability of biogas-to-hydrogen systems. With reforming processes requiring significant thermal input and purification demanding electrical power, thoughtful energy management can reduce operating costs by 30-50% compared to non-optimised systems. The interconnected nature of production stages creates numerous opportunities for heat recovery and process synergy.
Heat Recovery Systems That Cut Energy Costs
Strategic heat recovery transforms waste thermal energy into valuable process inputs, dramatically improving system efficiency. The high-temperature exhaust from reforming reactors (700-900°C) contains substantial energy that can preheat incoming biogas and steam streams, reducing primary energy requirements by 20-30%. Plate and shell heat exchangers with specialised high-temperature alloys effectively capture this thermal energy while resisting the corrosive compounds present in reformer exhaust.
Secondary recovery systems target medium-temperature waste heat from shift reactors and gas cooling stages (200-400°C). This energy tier ideally suits biogas preheating, digestate drying, or integration with organic Rankine cycle (ORC) systems that generate electricity from waste heat.
Combining Reforming Methods for Better Results
Hybrid reforming configurations combine complementary processes to optimise performance beyond what individual methods can achieve. The bi-reforming approach integrates steam and dry reforming by precisely controlling steam and CO₂ ratios, producing syngas with ideal H₂:CO ratios for subsequent applications while minimising carbon deposition issues. This approach is particularly well-suited to biogas, as it directly utilises both major components without separation.
Staged reforming configurations place different catalysts in series, with each optimised for specific reaction steps. A common arrangement employs a partial oxidation catalyst in the first stage to generate heat, followed by steam reforming and shift catalysts that utilise this thermal energy for endothermic reactions. This approach reduces external heating requirements by up to 40% compared to conventional steam reforming while maintaining high hydrogen yields.
Real-World Applications and Economic Viability
Biogas-to-hydrogen systems have transitioned from laboratory curiosities to commercial reality, with diverse applications demonstrating economic viability under specific conditions. The technology finds particular success where multiple value streams can be captured, including waste management credits, renewable energy incentives, and premium pricing for green hydrogen.
Fuel Cell Integration for Clean Electricity
Hydrogen fuel cells convert hydrogen back to electricity with exceptional efficiency, creating a renewable energy storage pathway with minimal environmental impact. Stationary fuel cell systems integrated with biogas reforming achieve electrical efficiencies of 45-60%—significantly higher than biogas combustion in engines or turbines. This configuration provides reliable baseload power while utilising waste heat for digester heating or other thermal applications, achieving overall system efficiencies exceeding 80%.

“Use of hydrogen: The Hydrogen Fuel Cell – U.S. Energy …” from www.eia.gov and used with no modifications.
Municipal wastewater treatment facilities represent ideal deployment sites, with many installations in Japan, South Korea, and California demonstrating successful operation. These facilities benefit from continuous biogas production, existing gas handling infrastructure, and substantial onsite electricity demand. The modular nature of fuel cell systems allows for incremental capacity expansion as biogas production increases or as financial resources become available.
Commercial viability typically requires capacity above 50 kW to offset fixed maintenance and control system costs. At this scale, the levelized cost of electricity ranges from $0.09-0.15/kWh without subsidies, competitive with retail electricity in many regions. With available incentives for renewable energy and waste reduction, numerous installations achieve payback periods of 4-7 years while delivering substantial carbon emission reductions compared to grid electricity.
Industrial Uses for Biogas-Derived Hydrogen
Industrial hydrogen consumption presents growing opportunities for biogas-derived hydrogen to replace fossil-based production. The food processing sector utilises hydrogen for hydrogenation reactions in edible oil production, with biogas-sourced hydrogen offering marketing advantages as a renewable processing input. Metallurgical applications, particularly in speciality metals production, value hydrogen's reducing properties and are increasingly willing to pay premiums for low-carbon alternatives to support sustainability goals.
Chemical manufacturing represents the largest potential market, with hydrogen serving as a fundamental building block for ammonia, methanol, and numerous other chemicals. While traditional chemical plants require massive hydrogen volumes, challenging for biogas-sourced production, distributed manufacturing models are emerging that align with biogas scale. These smaller facilities produce speciality chemicals closer to end users, reducing transportation costs while benefiting from renewable hydrogen's environmental attributes.
The transportation sector presents perhaps the most dynamic opportunity, with hydrogen fuel cell vehicles gaining traction, particularly in heavy-duty applications. Biogas-to-hydrogen fueling stations have emerged in several regions, typically co-located with landfills or wastewater treatment plants to minimise hydrogen transportation costs. Fleet operations with predictable routes and centralised fueling prove most suitable for early adoption, with transit agencies and waste collection services leading implementation.
“Hydrogen Production …” from www.mdpi.com and used with no modifications.
Cost Analysis: When Does Biogas Hydrogen Make Financial Sense?
Financial viability hinges on capturing price premiums for renewable hydrogen, which currently commands 20-100% higher prices than conventional hydrogen in environmentally conscious markets.
Carbon pricing mechanisms further improve economics, with each kilogram of biogas-derived hydrogen typically offsetting 8-12 kg of CO₂ compared to fossil-based alternatives.
Under favourable regulatory conditions with carbon prices exceeding $50/ton, biogas hydrogen may achieve cost parity with conventional production even without direct subsidies.
![]()
“biogas reforming …” from www.sciencedirect.com and used with no modifications.
Environmental Impact and Sustainability Benefits
Converting biogas to hydrogen creates cascading environmental benefits that extend far beyond simple carbon reduction.
This approach addresses multiple environmental challenges simultaneously, offering solutions to waste management issues while providing zero-emission fuel. The life cycle impacts vary significantly based on biogas source, production methods, and end-use applications.
Carbon Footprint Comparison with Fossil-Based Hydrogen
The greatest climate benefits occur when processing biogas from sources that would otherwise release methane directly to the atmosphere.
Landfill gas projects achieve the most favourable greenhouse gas balance, with each kilogram of hydrogen representing up to 15 kg CO₂e in avoided emissions when accounting for methane's potent global warming potential.
Agricultural waste digesters may follow closely, particularly those processing manure that would otherwise undergo anaerobic decomposition in lagoons or stockpiles.
Closing the Loop: Creating a Circular Energy Economy
Biogas-to-hydrogen systems exemplify circular economy principles by transforming waste streams into valuable energy carriers.
This approach closes multiple resource loops simultaneously—nutrients return to agricultural land through digestate application, energy embedded in organic matter is recovered as hydrogen, and carbon cycles through biological systems rather than being extracted from fossil deposits.
The localised nature of many biogas projects further enhances sustainability by reducing transportation impacts and strengthening regional resource security.
Future Innovations Changing the Hydrogen Landscape
The biogas-to-hydrogen sector stands at the threshold of transformative innovation, with emerging technologies promising to overcome current limitations in efficiency, scale, and economics. Research efforts focus on catalyst development, reactor design, and system integration to expand the practical applications of this renewable hydrogen pathway.
The convergence of advanced materials science, artificial intelligence for process optimisation, and modular manufacturing techniques is accelerating technology development cycles.
Many innovations that required decades to commercialise in traditional chemical processing are now moving from laboratory to market in 5-7 years, driven by urgent decarbonization imperatives and supportive policy frameworks.
Emerging Reforming Technologies to Watch
Plasma-assisted reforming represents one of the most promising innovations, using electrical energy to create non-thermal plasma that activates methane at much lower temperatures than conventional thermal processes.
This approach reduces energy requirements by 25-40% while enabling more compact reactor designs suitable for distributed applications. Early commercial systems have demonstrated effective operation with highly variable biogas compositions, addressing a key challenge for small-scale implementations.
Scaling Up: From Lab to Commercial Production
The pathway from laboratory discovery to commercial implementation involves overcoming significant engineering and economic hurdles. Successful scale-up strategies typically involve modular approaches that reduce risk through phased implementation while allowing for design optimisation based on operational experience.
Recent commercial deployments have demonstrated the value of standardised “skid-mounted” subsystems that can be factory-assembled and tested, reducing field construction costs by 15-30% compared to traditional build-in-place approaches.

Frequently Asked Questions About “Hydrogen Production from Biogas”
As biogas-to-hydrogen technology gains traction, project developers frequently encounter similar questions about implementation and performance. The following responses address common concerns while highlighting key considerations for successful deployment.
What biogas feedstocks produce the highest hydrogen yields?
Hydrogen yield correlates directly with methane content in the biogas, making feedstock selection crucial for maximising production.
Food waste digesters typically generate biogas with 65-75% methane content, yielding approximately 25-30% more hydrogen per cubic meter of biogas compared to landfill gas at 45-55% methane. This higher energy density translates directly to improved economics and smaller equipment footprints.
Beyond methane content, contaminant profiles significantly impact overall system performance.
How does biogas-derived hydrogen compare to green hydrogen from electrolysis?
Biogas-derived hydrogen and electrolytic hydrogen represent complementary approaches to renewable hydrogen production, each with distinct advantages. Some researchers suggest that biogas reforming will typically achieve slightly lower production costs and offer higher energy efficiency when accounting for the full energy chain.
However, electrolysis provides superior scalability, requires no carbon capture for net-zero emissions, and can directly utilise renewable electricity without conversion losses.
What government incentives exist for biogas-to-hydrogen projects?
Government support for biogas-to-hydrogen varies significantly by region but generally falls into three categories: production incentives, carbon credits, and capital subsidies. The U.S. Inflation Reduction Act established a clean hydrogen production tax credit of up to $3/kg for hydrogen with lifecycle emissions below 0.45 kg CO₂e/kg H₂, sufficient to make most biogas hydrogen projects economically competitive with fossil alternatives.
The European Union's Renewable Energy Directive II (RED II) classifies biogas-derived hydrogen as an advanced biofuel, making it eligible for premium pricing in transportation applications.
Many regional programs provide additional support, including California's Low Carbon Fuel Standard, which generates credits in the region of $5-15/kg of hydrogen, depending on carbon intensity. Capital grant programs targeting renewable hydrogen infrastructure have emerged in numerous jurisdictions, covering 20-50% of project costs for qualifying installations.
Regulatory frameworks continue evolving rapidly as governments implement hydrogen strategies aligned with climate commitments. The most successful projects typically engage early with relevant agencies to ensure eligibility for emerging incentive programs and to influence program design where possible.





