An anaerobic process is any biological or chemical process that occurs in the absence of oxygen. Read on for examples, including anaerobic respiration, where cells generate energy without oxygen by breaking down glucose into substances like lactic acid, and anaerobic digestion, where microorganisms break down organic waste to produce biogas.
Key Takeaways
- Anaerobic processes occur without oxygen, making them crucial for organisms in oxygen-limited environments.
- Glycolysis is the primary anaerobic process in cellular respiration, producing a small amount of ATP without requiring oxygen.
- While aerobic processes yield 36-38 ATP molecules per glucose molecule, anaerobic pathways produce only 2 ATP, demonstrating the efficiency difference.
- Your muscles switch to anaerobic metabolism during intense exercise, resulting in lactic acid buildup and the characteristic burning sensation.
- Anaerobic processes have critical applications in food production, waste management, and biofuel generation, showcasing their importance beyond basic cellular function.
Anaerobic Process: The Science of Living Without Oxygen
Life without oxygen might sound impossible, but it's happening inside your cells right now. Anaerobic processes are biological pathways that function without oxygen, and they're fundamental to everything from your high-intensity workout to the fermentation that creates your favorite foods. These oxygen-independent reactions power microorganisms in oxygen-depleted environments and provide emergency energy when your body can't get enough oxygen during intense physical activity.

What Makes a Process “Anaerobic”?
The term “anaerobic” comes from Greek roots: “an-” meaning “without” and “aero” referring to air or oxygen. When biologists describe a process as anaerobic, they're specifying that molecular oxygen (O₂) isn't required as an electron acceptor in that metabolic pathway. This distinction is crucial because it determines how organisms generate energy in different environments and situations.

“Hydrothermal vent – Wikipedia” from en.wikipedia.org and used with no modifications.
The Core Definition: Life Without Oxygen
At its most basic level, an anaerobic process is any biological reaction that occurs in the absence of oxygen. These processes have evolved as survival mechanisms in environments where oxygen is limited or nonexistent. Think about deep-sea thermal vents, waterlogged soils, or even the digestive tract of animals – all these oxygen-poor environments host thriving communities of anaerobic organisms.
Anaerobic metabolism typically relies on alternative electron acceptors or fermentation pathways to regenerate the NAD+ needed to continue energy production. While less efficient than aerobic respiration, these pathways allow life to persist in remarkable diversity across oxygen-deprived niches. Some microorganisms are obligate anaerobes, meaning oxygen is actually toxic to them, while others are facultative anaerobes that can switch between aerobic and anaerobic metabolism depending on environmental conditions. For more insight into how anaerobic processes work, you can explore anaerobic digestion and its applications.

“Microbial Life Without Oxygen has always been present below the surface in swamps and wetlands” from serc.carleton.edu and used with no modifications.
Difference Between Aerobic and Anaerobic Processes
The primary difference between aerobic and anaerobic processes lies in their relationship with oxygen. Aerobic processes require oxygen as the final electron acceptor in the electron transport chain, while anaerobic processes either use different electron acceptors or bypass the electron transport chain altogether. This distinction leads to significant differences in energy yield, efficiency, and byproducts.
Aerobic respiration is a complete oxidation process that breaks down glucose entirely into carbon dioxide and water while generating substantial ATP. In contrast, anaerobic pathways like fermentation result in partial breakdown of glucose, producing compounds like lactic acid or ethanol as byproducts. This incomplete oxidation explains why anaerobic processes yield far less energy than their oxygen-utilizing counterparts.
Comparison: Aerobic vs. Anaerobic Processes
Aerobic Process: Requires oxygen • Complete glucose breakdown • High energy yield (36-38 ATP) • End products: CO₂ and H₂O
Anaerobic Process: Functions without oxygen • Incomplete breakdown of glucose • Low energy yield (2 ATP) • End products vary (lactic acid, ethanol, etc.)
Energy Production Efficiency Comparison
When it comes to energy efficiency, aerobic processes are the clear winners. A single glucose molecule processed through aerobic respiration yields approximately 36-38 ATP molecules, providing substantial energy for cellular functions. By contrast, anaerobic glycolysis followed by fermentation produces only 2 ATP molecules per glucose – a mere fraction of the aerobic yield.
This efficiency gap explains why complex multicellular organisms like humans rely predominantly on aerobic metabolism for normal functioning. Our bodies switch to anaerobic pathways only during oxygen shortage situations, such as during sprinting or weightlifting, where oxygen demand exceeds supply. The low ATP yield of anaerobic processes also explains why these activities can't be sustained for long periods – they simply don't generate enough energy to meet ongoing demands.
Anaerobic Processes in Your Body
Your body relies on anaerobic processes more often than you might think. During normal cellular respiration, glycolysis—the first stage of glucose breakdown—occurs anaerobically in the cytoplasm before the oxygen-dependent stages begin. Additionally, when oxygen supply can't meet demand during intense exercise, your muscles shift to anaerobic metabolism to maintain energy production.
“What is Anaerobic Respiration? The …” from www.biologyonline.com and used with no modifications.
Glycolysis: The First Step of Cellular Respiration
Glycolysis serves as the initial stage of cellular respiration and operates completely independent of oxygen. This ancient metabolic pathway occurs in virtually all living organisms, breaking down glucose into two molecules of pyruvate while generating a modest 2 ATP and 2 NADH. The process takes place in the cytoplasm of cells and consists of ten enzyme-catalyzed reactions that transform a six-carbon glucose molecule into two three-carbon pyruvate molecules.
What's fascinating about glycolysis is its evolutionary significance – it likely evolved before oxygen was abundant in Earth's atmosphere, explaining why it doesn't require oxygen to function. This anaerobic process provided early life forms with energy before more efficient aerobic pathways emerged. Even today, glycolysis remains essential as the starting point for both aerobic and anaerobic energy production pathways.
Lactic Acid Fermentation During Exercise
When you push your body to its limits during high-intensity exercise like sprinting or heavy lifting, your muscles demand energy faster than oxygen can be delivered. This oxygen shortage forces your body to rely on anaerobic metabolism through lactic acid fermentation. This process allows glycolysis to continue by regenerating the NAD+ needed to keep the pathway functioning, albeit at the cost of producing lactic acid as a byproduct.
During lactic acid fermentation, the enzyme lactate dehydrogenase converts pyruvate (the end product of glycolysis) into lactic acid while simultaneously regenerating NAD+ from NADH. This recycling of NAD+ is crucial since glycolysis requires NAD+ to function, and without fermentation, the process would quickly grind to a halt in anaerobic conditions. The resulting lactic acid accumulation contributes to the burning sensation and fatigue experienced during intense exercise.
Why Your Muscles “Burn” During Intense Workouts
That familiar burning sensation during an intense workout is directly linked to anaerobic metabolism and lactic acid buildup. When your muscles operate anaerobically, lactic acid accumulates faster than your body can remove it, leading to increased acidity in your muscle tissue. This increased acidity interferes with enzyme function and muscle contractions, contributing to the burning sensation and eventual fatigue.
Contrary to popular belief, lactic acid isn't inherently bad – it's actually a valuable emergency fuel source that allows continued energy production when oxygen is limited. After exercise, your body recycles lactic acid through the Cori cycle, where it's transported to the liver and converted back to glucose. This process highlights how anaerobic metabolism serves as a crucial backup system when aerobic pathways can't meet energy demands.
Cellular Respiration and Anaerobic Pathways
Cellular respiration encompasses multiple interconnected pathways that extract energy from nutrients. While the complete process typically utilizes oxygen, the initial stage—glycolysis—operates anaerobically. This versatility allows cells to generate at least some ATP even when oxygen availability fluctuates, providing metabolic flexibility across diverse environmental conditions.
The Complete Cellular Respiration Process
Complete cellular respiration involves three main stages: glycolysis, the Krebs cycle (also called the citric acid cycle), and the electron transport chain. Only the first stage—glycolysis—can proceed anaerobically. The subsequent stages require oxygen to function properly, as oxygen serves as the final electron acceptor in the electron transport chain.
After glycolysis produces pyruvate in the cytoplasm, aerobic respiration continues with pyruvate entering the mitochondria. There, pyruvate is converted to acetyl-CoA, which enters the Krebs cycle to generate NADH and FADH2. These electron carriers then donate their electrons to the electron transport chain, where energy is harvested to pump protons across the inner mitochondrial membrane. This proton gradient drives ATP synthase to produce ATP, with oxygen serving as the final electron acceptor to form water.
Why Only Glycolysis Can Happen Without Oxygen
Glycolysis stands alone among cellular respiration pathways in its ability to function without oxygen because it doesn't involve redox reactions that require oxygen as an electron acceptor. Instead, glycolysis relies on substrate-level phosphorylation, where phosphate groups are directly transferred from high-energy intermediates to ADP to form ATP. This direct transfer mechanism doesn't require the elaborate electron transport system that depends on oxygen in later stages of respiration.
The Krebs cycle and electron transport chain, by contrast, fundamentally depend on oxygen to regenerate NAD+ and FAD. Without oxygen as the final electron acceptor, electrons would back up in the transport chain like cars in a traffic jam, halting energy production. Glycolysis avoids this dependency by employing fermentation pathways to regenerate NAD+ when oxygen is unavailable, allowing it to continue producing at least minimal ATP in anaerobic conditions.
ATP Production in Anaerobic vs. Aerobic Conditions
The ATP yield difference between anaerobic and aerobic metabolism is dramatic and explains why complex organisms rely predominantly on aerobic respiration. Anaerobic glycolysis followed by fermentation nets only 2 ATP molecules per glucose molecule—a remarkably inefficient energy extraction. This limited yield occurs because fermentation fails to harvest the considerable energy still locked in pyruvate and NADH after glycolysis.
Aerobic respiration, by contrast, extracts approximately 36-38 ATP molecules per glucose by fully oxidising the substrate through the Krebs cycle and electron transport chain. This 18-fold increase in energy yield explains why endurance activities rely on aerobic metabolism and why humans can sustain moderate exercise for hours but can only maintain all-out sprints for seconds. The dramatic efficiency difference also illustrates why most complex multicellular organisms evolved to depend primarily on oxygen-based metabolism.
Electron Transport Chain: Why It's Strictly Aerobic
The electron transport chain (ETC) represents the culmination of cellular respiration and is strictly dependent on oxygen. Located in the inner mitochondrial membrane, this series of protein complexes transfers electrons from NADH and FADH₂ through progressively more electronegative acceptors. This electron flow releases energy that pumps protons (H⁺) across the membrane, creating an electrochemical gradient that drives ATP synthesis.
Oxygen's unique role as the terminal electron acceptor makes it irreplaceable in the ETC. With its high electronegativity, oxygen readily accepts electrons, forming water as a harmless end product. Without oxygen, electrons would accumulate in the transport chain, preventing the regeneration of NAD⁺ and FAD needed for earlier steps in respiration. This explains why oxygen deprivation rapidly leads to cellular damage and why the ETC cannot function anaerobically.
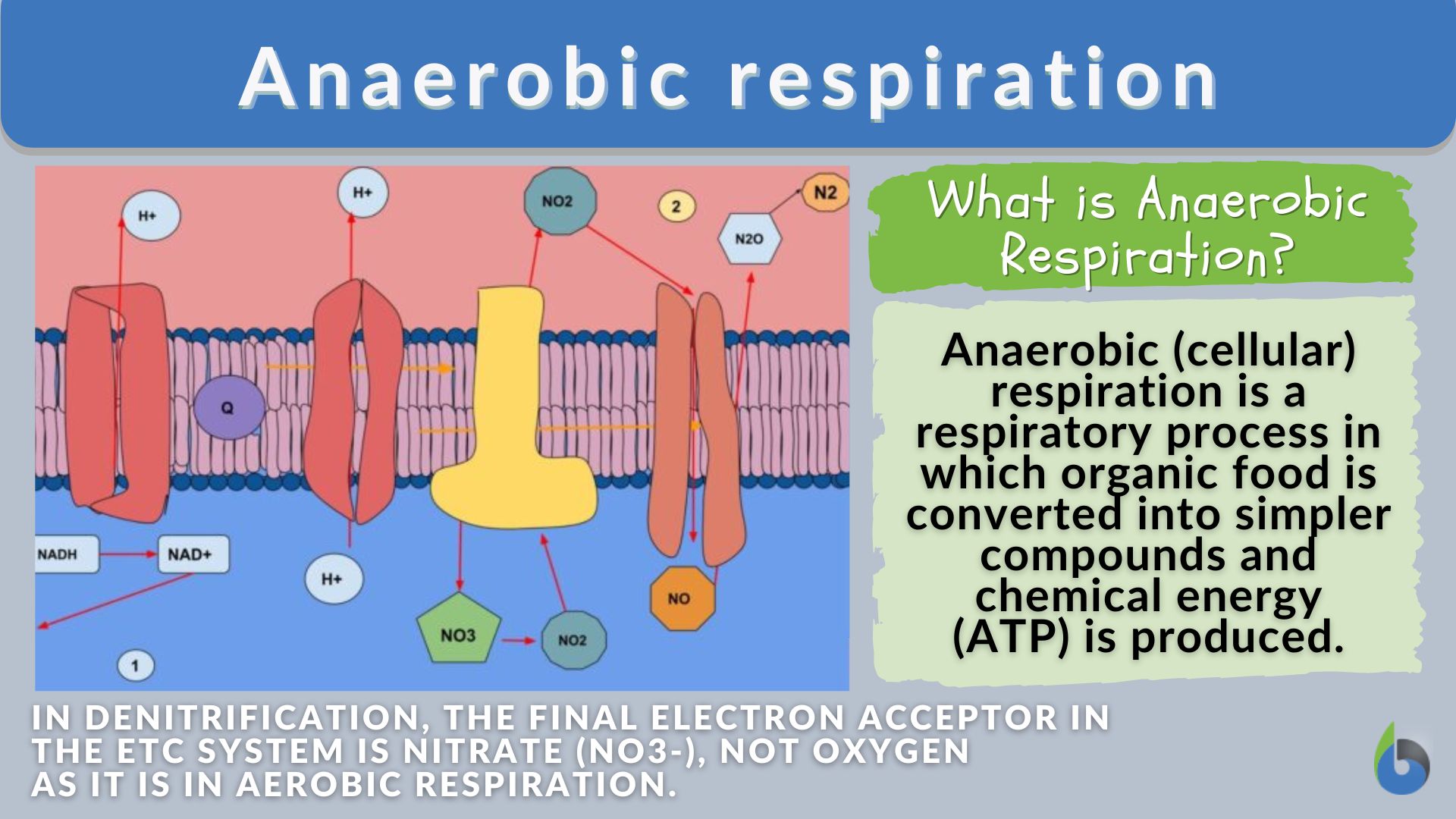
Some prokaryotes can perform anaerobic respiration using alternative terminal electron acceptors like nitrate, sulfate, or carbon dioxide instead of oxygen. However, these alternatives yield significantly less energy than oxygen-based respiration, highlighting oxygen's superior properties as an electron acceptor in energy metabolism. To understand more about this process, you can explore what is the anaerobic process.
Types of Anaerobic Processes in Nature
Nature has evolved a remarkable diversity of anaerobic processes that allow organisms to thrive in oxygen-depleted environments. From the alcoholic fermentation that produces our wines and beers to the methanogenesis occurring in swamp bottoms, these pathways represent ingenious solutions to the challenge of generating energy without oxygen. Understanding these processes provides insight into ecosystem functioning and has enabled numerous biotechnological applications.
Alcoholic Fermentation in Yeast
Alcoholic fermentation represents one of the most economically important anaerobic processes utilised by humans for thousands of years. This pathway, primarily carried out by yeast, converts pyruvate from glycolysis into ethanol and carbon dioxide. Saccharomyces cerevisiae, commonly known as baker's yeast, is the most widely used microorganism for this process, though many other fungi and some bacteria can perform similar fermentations.
During alcoholic fermentation, pyruvate is first decarboxylated (loses a carbon dioxide molecule) by the enzyme pyruvate decarboxylase to form acetaldehyde. Then, alcohol dehydrogenase reduces acetaldehyde to ethanol while oxidising NADH back to NAD+. This regeneration of NAD+ is crucial, as it allows glycolysis to continue functioning in anaerobic conditions. The byproducts of this process—ethanol and carbon dioxide—are what make alcoholic fermentation so valuable for bread making and alcoholic beverage production throughout human history.
Lactic Acid Fermentation in Bacteria
Lactic acid fermentation is another widely distributed anaerobic process performed by many bacteria, particularly those in the Lactobacillus, Streptococcus, and Lactococcus genera. These microorganisms convert pyruvate directly to lactic acid without producing carbon dioxide, using the enzyme lactate dehydrogenase to simultaneously regenerate the NAD+ needed for continued glycolysis. For more information on anaerobic processes, you can learn about biogas reactors and their role in energy production.
This anaerobic pathway is responsible for the production of numerous fermented foods, including yoghurt, cheese, sauerkraut, and kimchi. The lactic acid produced during fermentation not only creates the characteristic tangy flavour of these foods but also lowers the pH, inhibiting the growth of spoilage organisms and pathogens. Beyond food production, lactic acid bacteria play important roles in human health as members of the gut microbiome, where they produce beneficial compounds and help maintain intestinal homeostasis in the oxygen-limited environment of the digestive tract.
Methane Production in Swamps and Landfills
Methanogenesis represents a specialised form of anaerobic metabolism carried out exclusively by archaea known as methanogens. These microorganisms inhabit strictly anaerobic environments like swamp sediments, rice paddies, animal digestive tracts, and landfills. Unlike other anaerobic processes, methanogenesis doesn't involve fermentation but instead uses carbon dioxide as a terminal electron acceptor, reducing it to methane.
Methanogens operate at the end of anaerobic food chains, consuming the hydrogen, acetate, and other simple compounds produced by other anaerobes. This process is responsible for significant methane emissions from natural and human-made sources. While methane represents a potent greenhouse gas when released into the atmosphere, controlled methanogenesis is increasingly harnessed in biogas reactors to produce renewable energy, turning what could be an environmental liability into a valuable resource.
Real-World Applications of Anaerobic Processes
Anaerobic processes have been utilised by humans for millennia, often long before we understood the science behind them. Today, these oxygen-independent pathways are deliberately harnessed in numerous industries, from traditional food production to cutting-edge waste management and renewable energy generation. The ability of anaerobic microorganisms to function without oxygen makes them particularly valuable for processing organic materials in sealed, oxygen-free environments.
Food Production: Yoghurt, Cheese, and Bread
Many of our most beloved foods rely on anaerobic fermentation processes for their distinctive flavours, textures, and preservation. Yoghurt and cheese production depend on lactic acid bacteria performing anaerobic fermentation, converting lactose into lactic acid. This acidification not only creates tangy flavours but also causes milk proteins to coagulate, forming the characteristic curd structure of these dairy products.
Bread making similarly depends on anaerobic processes, with yeast performing alcoholic fermentation in the oxygen-limited environment of the dough. The carbon dioxide produced during fermentation creates the air pockets that give bread its light, airy texture, while the alcohol evaporates during baking. Beyond these examples, anaerobic fermentation is responsible for countless traditional foods worldwide, from sauerkraut and kimchi to salami and soy sauce, demonstrating how humans have harnessed these processes throughout culinary history.
Biofuel Generation
Anaerobic processes have emerged as critical components of renewable energy production, particularly in the generation of biofuels. Bioethanol production relies on yeast performing alcoholic fermentation to convert plant sugars into ethanol, which can be used as a renewable transportation fuel. This process allows agricultural residues and dedicated energy crops to be transformed into a liquid fuel compatible with existing infrastructure.
Similarly, biogas production harnesses the methanogenic capabilities of archaea to convert organic waste into methane-rich gas. Modern bioreactors provide optimised conditions for these anaerobic microorganisms, maximising methane yield from various feedstocks including food waste, agricultural residues, and dedicated energy crops. These applications demonstrate how understanding and controlling anaerobic processes can help address both waste management challenges and renewable energy needs simultaneously.
Wastewater Treatment
Anaerobic digestion plays a crucial role in modern wastewater treatment systems, particularly for processing high-strength organic wastes. Anaerobic digesters reduce the volume of sewage sludge while simultaneously producing biogas that can be captured and used for energy production. This approach offers significant advantages over aerobic treatment for certain waste streams, including lower energy requirements, reduced sludge production, and energy recovery potential.
The anaerobic treatment process typically involves multiple microbial communities working in syntrophy. First, hydrolytic bacteria break down complex organic compounds into simpler molecules. Acidogenic and acetogenic bacteria then convert these intermediates into acetate, hydrogen, and carbon dioxide. Finally, methanogenic archaea convert these products into methane gas. This cascading process effectively stabilises organic waste while generating a valuable energy product, exemplifying how anaerobic microbial communities can be harnessed for environmental benefit.
Anaerobic Digestion for Waste Management
Anaerobic digestion has emerged as a cornerstone technology for sustainable organic waste management. This controlled biological process breaks down biodegradable materials in oxygen-free environments, reducing waste volume while producing biogas containing approximately 60% methane. Modern anaerobic digestion facilities process diverse feedstocks, including food waste, agricultural residues, manure, and dedicated energy crops. Learn more about the anaerobic process and its applications.
The technology offers multiple environmental benefits beyond energy production, including reduced landfill use, decreased methane emissions from decomposing organic waste, and production of nutrient-rich digestate that can be used as fertiliser. Many municipalities and food processing facilities now incorporate anaerobic digestion into their waste management strategies, transforming what was once considered waste into valuable resources while closing nutrient cycles and reducing environmental impacts.
When Anaerobic Processes Become a Problem
While anaerobic processes provide numerous benefits in controlled settings, they can also create significant challenges when they occur in unintended contexts. Anaerobic conditions in normally aerobic environments often signal disrupted ecosystems or disease states. Understanding when and how these processes become problematic helps in developing preventative measures and appropriate interventions.
In environmental contexts, uncontrolled anaerobic decomposition can release methane—a potent greenhouse gas—into the atmosphere, contributing to climate change. In medical settings, anaerobic infections can be particularly challenging to treat due to their location in oxygen-poor tissues and their frequent resistance to certain antibiotics. These examples highlight how the same metabolic versatility that makes anaerobic organisms valuable in some contexts can create significant problems in others. For more information on anaerobic processes, you can explore anaerobic digestion and its capacity in the UK.
The balance between beneficial and problematic anaerobic processes often comes down to control and context. Harnessing these pathways requires creating appropriate containment, optimising conditions for desired outcomes, and preventing unintended consequences. This balance exemplifies the broader principle that microbial processes are neither inherently good nor bad—their impact depends on where, when, and how they occur.
Anaerobic Infections and Diseases
Anaerobic infections represent a significant medical challenge, occurring when anaerobic bacteria colonise normally sterile, oxygen-poor body sites. These infections often develop in deep tissues, abscesses, or after traumatic injuries where blood supply and oxygen levels are compromised. Common anaerobic pathogens include Clostridium, Bacteroides, Prevotella, and Peptostreptococcus species, which thrive in the oxygen-depleted conditions of damaged tissues.
![]()
“Anaerobic bacteria” from www.ucsfhealth.org and used with no modifications.
What makes anaerobic infections particularly concerning is their tendency to cause tissue destruction, gas production, and foul-smelling discharge. Certain anaerobic pathogens produce potent toxins, with Clostridium tetani (causing tetanus) and Clostridium botulinum (causing botulism) representing extreme examples of how anaerobic metabolism can generate highly dangerous compounds. Treatment typically involves surgical debridement to remove dead tissue, antibiotics effective against anaerobic organisms, and measures to increase oxygen availability in the affected tissues.
![]()
“Anaerobic Infections and Endocrinology …” from www.ncbi.nlm.nih.gov and used with no modifications.
Environmental Issues from Methane Production
Methane production through anaerobic decomposition creates significant environmental challenges when uncontrolled. As a greenhouse gas, methane is approximately 25 times more potent than carbon dioxide over a 100-year period, making its release from landfills, rice paddies, and livestock production major contributors to climate change. This potent warming effect occurs because methane absorbs infrared radiation more effectively than carbon dioxide, trapping more heat in the atmosphere.
Human activities have dramatically increased anaerobic methane production through expanded rice cultivation, increased livestock numbers, and improper waste management. Efforts to mitigate these emissions include capturing landfill gas for energy production, improving manure management systems, and developing modified rice cultivation techniques that reduce anaerobic decomposition. These strategies aim to transform problematic anaerobic methane production into controlled processes that can provide renewable energy while reducing greenhouse gas impacts.
Why Understanding Anaerobic Processes Matters
Understanding anaerobic processes provides fundamental insights into how life functions in oxygen-limited environments and offers practical applications across multiple fields. From explaining why your muscles fatigue during intense exercise to informing climate change mitigation strategies, knowledge of these oxygen-independent pathways connects basic biology to everyday phenomena and global challenges. By recognising how and why organisms can function without oxygen, we gain a more complete picture of life's remarkable metabolic diversity and the evolutionary adaptations that enable survival in challenging conditions.
Frequently Asked Questions – About What Does it Mean if a Process is Anaerobic
The following questions address common points of confusion about anaerobic processes, providing clear, science-based explanations for these important biological phenomena. These answers help bridge the gap between technical biochemistry and everyday observations, from exercise physiology to food production.
Can humans survive in completely anaerobic conditions?
No, humans cannot survive in completely anaerobic conditions. While our cells can temporarily produce energy anaerobically through glycolysis and lactic acid fermentation, these pathways generate insufficient ATP to sustain life and result in metabolic acidosis when prolonged. Our brain, heart, and other vital organs require the high ATP yield of aerobic metabolism to function properly. When oxygen supply is completely cut off, brain cells begin dying within minutes, highlighting our fundamental dependence on oxygen-based energy production.
What happens to muscles during anaerobic exercise?
During high-intensity, anaerobic exercise, muscle cells shift from primarily aerobic metabolism to increasing reliance on anaerobic glycolysis and lactic acid fermentation. This metabolic shift occurs because oxygen demand exceeds supply, forcing cells to generate ATP without oxygen. The process produces lactic acid as a byproduct, which accumulates in muscle tissue and enters the bloodstream.
This lactic acid accumulation contributes to the burning sensation, fatigue, and eventual inability to maintain high-intensity effort. However, contrary to common misconception, lactic acid isn't simply a waste product—it's an important alternative fuel source during oxygen limitation. After exercise, your body converts much of this lactic acid back to glucose in the liver through the Cori cycle, demonstrating how anaerobic metabolism serves as a valuable, if temporary, energy-producing strategy.
Why does fermentation produce alcohol in some cases and lactic acid in others?
The end products of fermentation—whether ethanol or lactic acid—depend on the specific enzymes possessed by the organism performing the process. Yeast and certain bacteria have pyruvate decarboxylase and alcohol dehydrogenase, which convert pyruvate to ethanol via acetaldehyde. In contrast, lactic acid bacteria and human muscle cells contain lactate dehydrogenase, which directly converts pyruvate to lactic acid. These different enzymatic pathways evolved separately but serve the same core purpose: regenerating NAD+ to allow continued ATP production through glycolysis under anaerobic conditions.
Are all bacteria capable of anaerobic respiration?
No, not all bacteria can perform anaerobic respiration. Bacteria exhibit diverse metabolic capabilities that reflect their evolutionary adaptations to specific environments. Obligate aerobes, like Mycobacterium tuberculosis, strictly require oxygen and cannot grow anaerobically. Obligate anaerobes, such as Clostridium botulinum, cannot tolerate oxygen and die when exposed to it. Facultative anaerobes, including Escherichia coli, can switch between aerobic and anaerobic metabolism depending on oxygen availability.
Additionally, bacteria vary in which alternative electron acceptors they can use for anaerobic respiration. Some can use nitrate, sulfate, iron, carbon dioxide, or other compounds as terminal electron acceptors when oxygen is unavailable. This metabolic diversity explains why bacteria can colonise virtually every environment on Earth, from oxygen-rich surface waters to deep subsurface habitats completely devoid of oxygen. For instance, the use of carbon dioxide as an electron acceptor is a crucial process in biomethane production.
How do scientists study anaerobic organisms if oxygen is everywhere?
Scientists have developed specialised equipment and techniques to create and maintain oxygen-free conditions for studying anaerobic organisms. Anaerobic chambers (glove boxes) provide sealed environments where researchers can manipulate samples in oxygen-free atmospheres, typically consisting of nitrogen, carbon dioxide, and hydrogen. These chambers include airlock systems for transferring materials without introducing oxygen and often contain palladium catalysts that combine any trace oxygen with hydrogen to form water.
For culturing anaerobic microorganisms, scientists use media containing reducing agents like thioglycolate or cysteine that scavenge oxygen. Resazurin, an indicator dye that changes colour in the presence of oxygen, is often added to verify anaerobic conditions. Specialised techniques like Hungate's method and its modifications enable the preparation and maintenance of strictly anaerobic cultures. These approaches have been essential for studying the estimated 99% of microbial species that cannot be cultured under standard aerobic laboratory conditions.





