Different Processes, Reactions, and Uses for Methane Combustion
Key Takeaways
- Methane is a significant energy source, powering homes and industries worldwide.
- Complete combustion of methane releases more energy and fewer pollutants than incomplete combustion.
- Incomplete combustion can lead to harmful byproducts such as carbon monoxide.
- Controlled combustion in industrial settings optimizes energy output and reduces emissions.
- Understanding methane combustion can help develop cleaner energy solutions.
Methane Combustion: Basics and Importance
Methane combustion is a fundamental process in energy production. When methane burns, it reacts with oxygen to produce carbon dioxide, water, and energy. This reaction is critical for generating electricity, heating homes, and powering industrial processes. Methane's efficiency and relatively low emissions make it a preferred choice among fossil fuels.
“Balanced Equation for the Combustion of …” from www.youtube.com and used with no modifications.
Why Methane Matters in Energy Production
Methane is the main component of natural gas, which is a major energy source globally. It's cleaner than coal and oil, producing less carbon dioxide per unit of energy released. This makes it an attractive option for reducing greenhouse gas emissions while meeting energy demands. In power plants, methane is burned in turbines to generate electricity efficiently.
Environmental Impact of Methane Combustion
While methane combustion is cleaner than burning other fossil fuels, it still impacts the environment. The primary concern is carbon dioxide emissions, a significant contributor to global warming. However, methane itself is a potent greenhouse gas, and leaks during extraction and transportation can exacerbate climate change. For further insights into the benefits of methane, explore the advantages of biomethane plants.
“Methane has over 80 times the warming power of carbon dioxide over the first 20 years after it reaches the atmosphere.”
Reducing methane leaks and improving combustion efficiency are crucial for minimizing its environmental impact. Innovations in capture technology and stricter regulations can help achieve this goal.
Methane's Role in Clean Energy Solutions
Methane is not just a transitional fuel; it's also part of the clean energy future. With advancements in carbon capture and storage (CCS) technology, methane combustion can become even cleaner. Additionally, biogas, a renewable form of methane, can be produced from organic waste, providing a sustainable energy source.
By focusing on methane's role in clean energy, we can reduce our carbon footprint while maintaining energy security. Transitioning to biogas and integrating CCS technologies are essential steps toward a more sustainable future.
![]()
“Combustion of Methane bubbles” from www2.chem.wisc.edu and used with no modifications.
Types of Methane Combustion
Understanding the types of methane combustion is crucial for optimizing its use. There are primarily two types: complete and incomplete combustion. Each has different conditions and outcomes, affecting energy efficiency and emissions. For more details on the interaction of methane with oxygen, you can explore further resources.
Complete Combustion: Ideal Conditions
“Complete combustion occurs when there is enough oxygen to allow the fuel to react completely.”
In complete combustion, methane reacts fully with oxygen, producing carbon dioxide, water, and maximum energy output. This process is efficient and minimizes pollutants. The ideal conditions include sufficient oxygen supply and proper temperature control. For more information on the benefits of biogas production, explore the advantages of biomethane plants.
For example, in a well-designed gas stove, complete combustion ensures efficient cooking with minimal soot or carbon monoxide emissions. This not only saves energy but also enhances safety in domestic settings.
Complete combustion is desirable in industrial applications as well. It maximizes energy production while reducing harmful emissions, making it a preferred choice for power generation.
Chemical Reactions Involved
The combustion of methane involves a series of chemical reactions that convert methane and oxygen into carbon dioxide, water, and energy. These reactions are exothermic, meaning they release energy, which is harnessed for various applications. Understanding these reactions is key to improving efficiency and reducing emissions. For more insights into how methane is utilized, explore the advantages of biomethane plants.
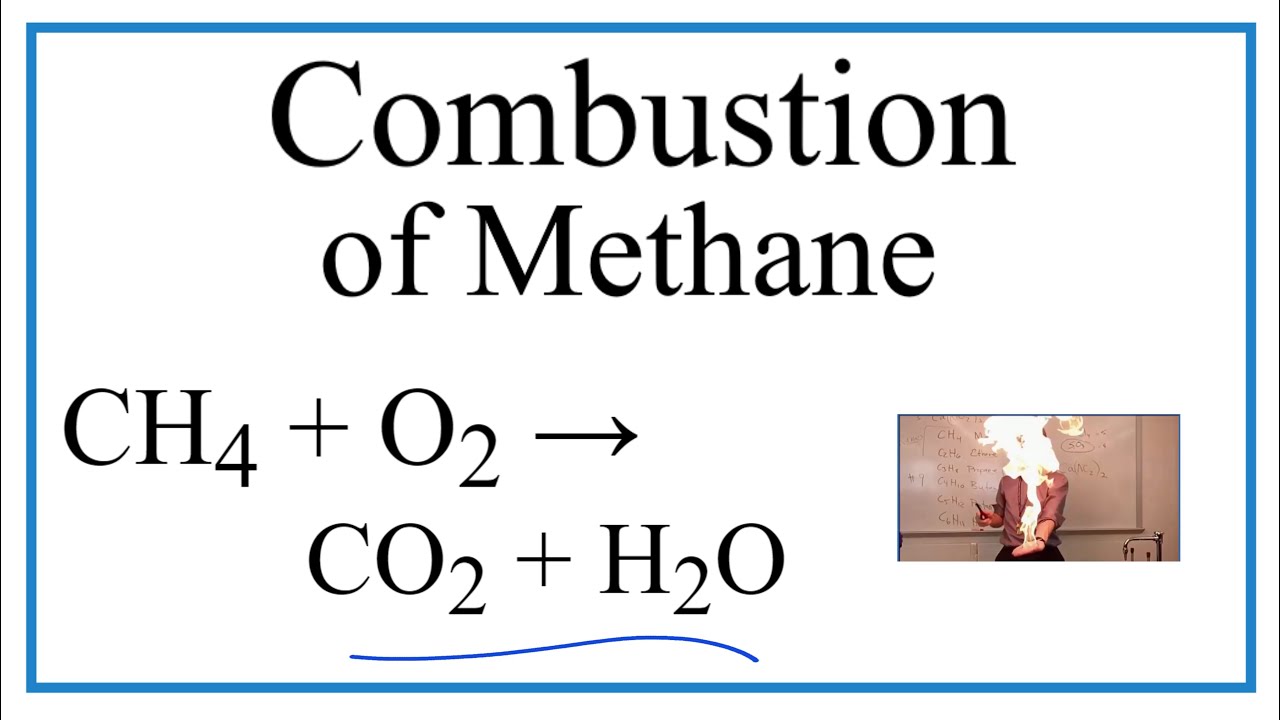
Equation of Methane Combustion
The basic chemical equation for methane combustion is:
CH4 + 2O2 → CO2 + 2H2O + energy
This equation shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. The reaction releases a significant amount of energy, which is why methane is such a valuable energy source.
Role of Oxygen in the Reaction
Oxygen plays a critical role in methane combustion. Adequate oxygen supply ensures complete combustion, maximizing energy output and minimizing harmful byproducts. Insufficient oxygen can lead to incomplete combustion, producing carbon monoxide and soot, which are both hazardous to health and the environment.
In industrial settings, controlling the oxygen supply is crucial for optimizing combustion efficiency. Advanced technologies, such as oxygen sensors and automated control systems, help maintain the ideal oxygen levels, ensuring safe and efficient combustion.

“oxygen – combustion reaction …” from melscience.com and used with no modifications.
Heat and Energy Output
The heat and energy output from methane combustion are substantial. Methane's high energy content makes it an efficient fuel for generating electricity and heat. The energy released during combustion can be harnessed in various ways, from powering turbines to heating homes.
For instance, in a gas-fired power plant, methane combustion drives turbines that generate electricity. The heat produced can also be used in combined heat and power (CHP) systems, which improve overall energy efficiency by utilizing both electricity and heat.
Applications of Methane Combustion
Methane combustion has a wide range of applications, from domestic energy use to large-scale industrial processes. Its versatility and efficiency make it a popular choice for many energy needs.
Methane as a Domestic Energy Source
In homes, methane is commonly used for cooking and heating. Natural gas stoves and heaters rely on methane combustion to provide a convenient and efficient energy source. Complete combustion in these appliances ensures safe and effective use, minimizing emissions and energy waste.
Use in Power Generation
Methane is a key fuel for power generation. Gas-fired power plants use methane to produce electricity, offering a cleaner alternative to coal-fired plants. These plants are capable of ramping up quickly to meet peak demand, making them an essential part of the energy grid.
The efficiency and lower emissions of methane combustion make it an attractive option for reducing the carbon footprint of electricity generation. With the integration of carbon capture and storage technologies, methane can play a significant role in transitioning to cleaner energy systems.
Industrial Applications
Industries utilize methane combustion for various processes, including heating, drying, and steam generation. The high energy output and reliability of methane make it suitable for demanding industrial applications.
For example, in the chemical industry, methane is used as a feedstock for producing hydrogen and other chemicals. Its combustion provides the necessary heat and energy for these processes, supporting the production of essential industrial materials.

Advancements in Methane Use
Recent advancements in methane use focus on improving efficiency and reducing environmental impact. Innovations such as biogas production, which involves generating methane from organic waste, provide renewable and sustainable energy solutions.
Moreover, developments in carbon capture and storage (CCS) technologies are enhancing the environmental performance of methane combustion. By capturing and storing carbon emissions, these technologies help mitigate the impact of methane use on climate change.
Overall, methane's versatility and potential for cleaner combustion make it a vital component of modern energy systems. By continuing to innovate and optimize its use, we can harness its benefits while minimizing its environmental footprint. For instance, understanding the purposes and design of biogas compressors can significantly enhance methane utilization.
Final Thoughts on Methane Combustion
Methane combustion is a cornerstone of modern energy systems, providing a reliable and efficient source of power. Its ability to produce significant energy while emitting fewer pollutants than other fossil fuels makes it an essential component of both current and future energy landscapes. However, to fully harness its potential, it's crucial to address the challenges associated with methane use and explore innovative solutions for cleaner combustion.
Future Prospects and Innovations
The future of methane combustion lies in its integration with renewable energy sources and advancements in technology. Innovations such as biogas production and carbon capture and storage (CCS) offer promising pathways for reducing the environmental impact of methane use. By converting organic waste into biogas, we can create a sustainable energy source that complements traditional methane combustion.
Furthermore, ongoing research into CCS technologies aims to capture and store carbon emissions from methane combustion, effectively reducing its contribution to climate change. These innovations, along with improved combustion efficiency and stricter regulations, can pave the way for a cleaner and more sustainable energy future.
Challenges and Solutions
Despite its advantages, methane combustion faces several challenges, particularly concerning its environmental impact. Methane leaks during extraction and transportation can significantly contribute to greenhouse gas emissions. Addressing these leaks is crucial for minimizing methane's environmental footprint.
Moreover, incomplete combustion can produce harmful byproducts such as carbon monoxide and soot. To combat this, industries must prioritize efficient combustion processes and implement advanced technologies to ensure complete combustion. By doing so, we can maximize energy output while minimizing emissions. For instance, utilizing a biogas compressor can help optimize the combustion process and reduce harmful emissions.
Ultimately, a combination of technological advancements, regulatory measures, and public awareness is needed to overcome these challenges and optimize methane use. As we continue to explore and develop cleaner energy solutions, methane will play a vital role in the transition to a sustainable energy future.
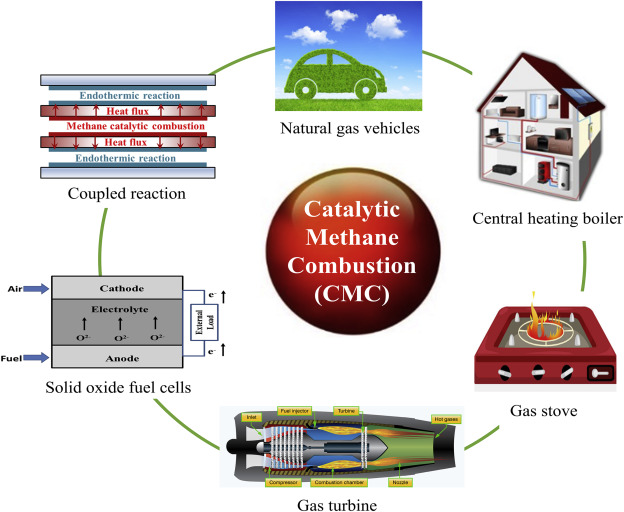
“catalytic methane combustion …” from www.sciencedirect.com and used with no modifications.
Frequently Asked Questions
Here are some common questions about methane combustion and its implications for energy and the environment.
What makes methane a preferred fuel?
Methane is favored as a fuel due to its high energy content and lower emissions compared to other fossil fuels. It burns more cleanly, producing less carbon dioxide per unit of energy released, making it an efficient and environmentally friendly choice for power generation and heating.
How does methane combustion impact the environment?
While methane combustion produces fewer emissions than other fossil fuels, it still contributes to greenhouse gas emissions through the release of carbon dioxide. Methane leaks during extraction and transportation also pose environmental risks, as methane is a potent greenhouse gas.
To mitigate these impacts, it's essential to improve methane capture and reduce leaks throughout the supply chain. Additionally, advancements in combustion technology can help minimize emissions and enhance efficiency.
- Implementing carbon capture and storage (CCS) technologies can further reduce the environmental impact of methane combustion.
- Transitioning to renewable biogas can provide a sustainable alternative to traditional methane sources.
What are the byproducts of methane combustion?
The primary byproducts of complete methane combustion are carbon dioxide and water. However, incomplete combustion can produce carbon monoxide and soot, which are harmful to health and the environment. Ensuring adequate oxygen supply and optimal combustion conditions can minimize these byproducts.
How is methane combustion managed in power plants?
In power plants, methane combustion is carefully managed to optimize efficiency and reduce emissions. Advanced technologies such as oxygen sensors and automated control systems help maintain ideal combustion conditions, ensuring complete combustion and minimizing harmful byproducts.
Additionally, many power plants are integrating carbon capture and storage technologies to further reduce their carbon footprint and improve environmental performance.
Can methane be used as an alternative to other fossil fuels?
Yes, methane can serve as an alternative to other fossil fuels, particularly coal and oil. Its cleaner combustion profile and high energy content make it an attractive option for reducing greenhouse gas emissions while meeting energy demands. By transitioning to methane and incorporating renewable biogas, we can create a more sustainable and environmentally friendly energy system.
As we continue to innovate and improve methane use, it will remain a vital component of the global energy mix, supporting the transition to a cleaner and more sustainable future.





